Before science was able to shed light on human reproduction, most people thought new life arose through spontaneous generation from non-living matter. That changed a smidgen in the middle of the 17th century, when natural philosophers were able (barely) to see the female ovum, or egg, with the naked eye. They theorised that all life was spawned at the moment of divine creation; one person existed inside the other within a woman’s eggs, like Russian nesting dolls. This view of reproduction, called preformation, suited the ruling class well. ‘By putting lineages inside each other,’ notes the Portuguese developmental biologist and writer Clara Pinto-Correia in The Ovary of Eve (1997), ‘preformation could function as a “politically correct” antidemocratic doctrine, implicitly legitimising the dynastic system – and of course, the leading natural philosophers of the Scientific Revolution certainly were not servants.’
One might think that, as science progressed, it would crush the Russian-doll theory through its lucid biological lens. But that’s not precisely what occurred – instead, when the microscope finally enabled researchers to see not just eggs but sperm, the preformation theory morphed into a new, even more patriarchal political conceit: now, held philosophers and some students of reproduction, the egg was merely a passive receptacle waiting for vigorous sperm to arrive to trigger development. And sperm? The head of each contained a tiny preformed human being – a homunculus, to be exact. The Dutch mathematician and physicist Nicolaas Hartsoeker, inventor of the screw-barrel microscope, drew his image of the homunculus when sperm became visible for the first time in 1695. He did not actually see a homunculus in the sperm head, Hartsoeker conceded at the time, but he convinced himself that it was there.
More powerful microscopes eventually relegated the homunculus to the dustbin of history – but in some ways not much has changed. Most notably, the legacy of the homunculus survives in the stubbornly persistent notion of the egg as a passive participant in fertilisation, awaiting the active sperm to swim through a hailstorm of challenges to perpetuate life. It’s understandable – though unfortunate – that a lay public might adopt these erroneous, sexist paradigms and metaphors. But biologists and physicians are guilty as well.
It was in the relatively recent year of 1991, long after much of the real science had been set in stone, that the American anthropologist Emily Martin, now at New York University, described what she called a ‘scientific fairy tale’ – a picture of egg and sperm that suggests that ‘female biological processes are less worthy than their male counter-parts’ and that ‘women are less worthy than men’. The ovary, for instance, is depicted with a limited stock of starter eggs depleted over a lifetime whereas the testes are said to produce new sperm throughout life. Human egg production is commonly described as ‘wasteful’ because, from 300,000 egg starter cells present at puberty, only 400 mature eggs will ever be released; yet that adjective is rarely used to describe a man’s lifetime production of more than 2 trillion sperm. Whether in the popular or scientific press, human mating is commonly portrayed as a gigantic marathon swimming event in which the fastest, fittest sperm wins the prize of fertilising the egg. If this narrative was just a prejudicial holdover from our sexist past – an offensive male fantasy based on incorrect science – that would be bad enough, but continued buy-in to biased information impedes crucial fertility treatments for men and women alike.
To grasp how we got here, a tour through history can help. Scientific understanding of sex cells and the process of human conception is a comparatively recent development. An egg, the largest cell in a human body, is barely visible to the naked eye, and about as big as the period ending this sentence. So the smallest human body cell, a sperm, is utterly invisible for the unaided eye.
Sperm were unknown to science until 1677, when the Dutch amateur scientist Antonie van Leeuwenhoek first observed human sperm under a microscope. Around the same time, it was realised that the human ovary produced eggs, although it was not until 1827 that the German biologist Karl Ernst von Baer first reported actual observations of human and other mammalian eggs.
After van Leeuwenhoek’s discovery of sperm, it took another century before anyone realised that they were needed to fertilise eggs. That revelation came in the 1760s, when the Italian priest and natural scientist Lazzaro Spallanzani, experimenting on male frogs wearing tight-fitting taffeta pants, demonstrated that eggs would not develop into tadpoles unless sperm was shed into the surrounding water. Bizarrely, until Spallanzani announced his findings, it was widely thought – even by van Leeuwenhoek for some years – that sperm were tiny parasites living in human semen. It was only in 1876 that the German zoologist Oscar Hertwig demonstrated the fusion of sperm and egg in sea urchins.
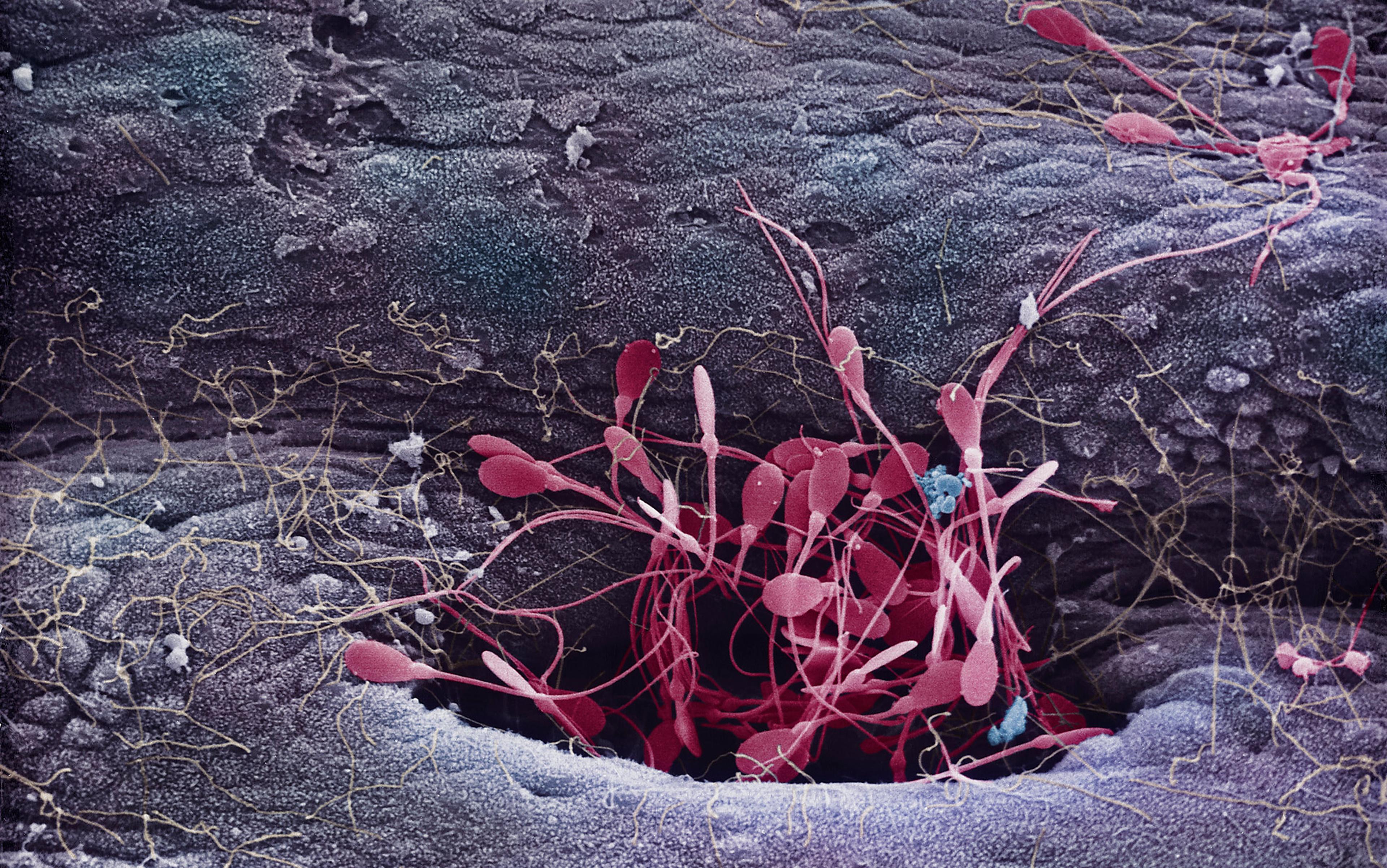
Eventually, powerful microscopes revealed that an average human ejaculate, with a volume of about half a teaspoon, contains some 250 million sperm. But a key question remains unanswered: ‘Why so many?’ In fact, studies show that pregnancy rates tend to decline once a man’s ejaculate contains less than 100 million sperm.
Clearly, then, almost half the sperm in an average human ejaculate are needed for normal fertility. A favoured explanation for this is sperm competition, stemming from that macho-male notion of sperm racing to fertilise – often with the added contention that more than one male might be involved. As in a lottery, the more tickets you buy, the likelier you are to win. Natural selection, the thinking goes, drives sperm numbers sky-high in a kind of arms race for the fertilisation prize.
Striking examples of sperm competition do indeed abound in the animal kingdom. Our closest relatives, the chimpanzees, live in social units containing several adult males that regularly engage in promiscuous mating; females in turn are mated by multiple males. Numerous features, such as conspicuously large testes, reflect a particularly high level of sperm production in such mammal species. In addition to large testes, they have fast sperm production, high sperm counts, large sperm midpieces (containing numerous energy-generating mitochondria for propulsion), notably muscular sperm-conducting ducts, large seminal vesicles and prostate glands, and high counts of white blood cells (to neutralise sexually transmitted pathogens). The vesicles and the prostate gland together produce seminal fluid, which can coagulate to form a plug in the vagina, temporarily blocking access by other males.
Popular opinion and even many scientists perpetuate the same sperm scenario for humans, but evidence points in a different direction. In fact, despite various lurid claims to the contrary, there’s no convincing evidence that men are biologically adapted for sperm competition. The story of sperm abundance in promiscuously mating chimpanzees contrasts with what we see in various other primates, including humans. Many primates live in groups with just a single breeding male, lack direct competition and have notably small testes. In all relevant comparisons, humans emerge as akin to primates living in single-male groups – including the typical nuclear family. Walnut-sized human testes are just a third of the size of chimpanzee testes, which are about as large chickens’ eggs. Moreover, while chimpanzee ejaculate contains remarkably few physically abnormal sperm, human semen contains a large proportion of duds. Quality controls on human ejaculate have seemingly been relaxed in the absence of direct sperm competition.
Sperm passage is more like a challenging military obstacle course than a standard swimming race
For species not regularly exposed to direct sperm competition, the only promising alternative explanation for high sperm counts concerns genetic variation. In a couple of rarely cited papers published more than four decades ago, the biologist Jack Cohen at the University of Birmingham in the UK noted an association between sperm counts and the generation of chromosome copies during sperm production. During meiosis, the special type of cell division that produces sex cells, pairs of chromosomes exchange chunks of material through crossing over. What Cohen found is that, across species, sperm counts increase in tandem with the number of crossovers during their production. Crossing over increases variation, the essential raw material for natural selection. Think of sperm production as a kind of lottery in which enough tickets (sperm) are printed to match available numbers (different genetic combinations).
Other findings fly in the face of the popular scenario, too. For instance, most mammalian sperm do not in fact swim up the entire female tract but are passively transported part or most of the way by pumping and wafting motions of the womb and oviducts. Astoundingly, sperm of smaller mammals tend to be longer on average than sperm of larger mammals – a mouse sperm is longer than the sperm of a whale. But even if these were equivalent in size, swimming up to an egg becomes more of a stretch the larger a species gets. Indeed, it might be feasible for a mouse sperm to swim all the way up to the egg – but it is quite impossible for an even smaller blue whale sperm to swim 100 times further up the female tract unaided. Convincing evidence has instead revealed that human sperm are passively transported over considerable distances while travelling through the womb and up the oviducts. So much for Olympic-style racing sperm!
In fact, of the 250 million sperm in the average human ejaculate, only a few hundred actually end up at the fertilisation site high up in the oviduct. Sperm passage up the female tract is more like an extremely challenging military obstacle course than a standard sprint-style swimming race. Sperm numbers are progressively whittled down as they migrate up the female tract, so that less than one in a million from the original ejaculate will surround the egg at the time of fertilisation. Any sperm with physical abnormalities are progressively eliminated along the way, but survivors surrounding the egg are a random sample of intact sperm.
Many sperm do not even make it into the neck of the womb (cervix). Acid conditions in the vagina are hostile and sperm do not survive there for long. Passing through the cervix, many sperm that escape the vagina become ensnared in mucus. Any with physical deformities are trapped. Moreover, hundreds of thousands of sperm migrate into side-channels, called crypts, where they can be stored for several days. Relatively few sperm travel directly though the womb cavity, and numbers are further reduced during entry into the oviduct. Once in the oviduct, sperm are temporarily bound to the inner surface, and only some are released and allowed to approach the egg.
Pushing the notion that the fertilising sperm is some kind of Olympic champion has obscured the fact that an ejaculate can contain too many sperm. If sperm surround the egg in excessive numbers, the danger of fertilisation by more than one (polyspermy) arises with catastrophic results. Polyspermy occasionally occurs in humans, especially when fathers have very high sperm counts. In the commonest outcome in which two sperm fertilise an egg, cells of the resulting embryo contain 69 chromosomes instead of the usual 46. This is always fatal, usually resulting in miscarriage. Although some individuals survive as far as birth, they always expire shortly afterwards. Because polyspermy typically has a fatal outcome, evolution has evidently led to a series of obstacles in the female reproductive tract that strictly limit the number of sperm allowed to surround an egg.
Polyspermy has practical implications for assisted reproduction in cases of compromised fertility or infertility. For instance, the original standard procedure of introducing semen into the vagina for artificial insemination has been replaced by direct injection into the womb (intrauterine insemination, or IUI). Directly introducing semen into the womb bypasses the reduction of sperm numbers that normally occurs in the cervix, where mucus weeds out physically abnormal sperm. Analyses of clinical data have revealed that depositing 20 million sperm in the womb (less than a 10th of the number in the average ejaculate) is enough to achieve a routine pregnancy rate.
Sperm numbers become even more important when it comes to in vitro fertilisation (IVF), with direct exposure of an egg to sperm in a glass vessel. This bypasses every single one of the natural filters between the vagina and the egg. In the early development of IVF, the general tendency was to use far too many sperm. This reflected the understandable aim of maximising fertilisation success, but it ignored natural processes. High sperm numbers between 50,000 and 0.5 million increasingly depressed the success rate. Optimal fertilisation rates were achieved with only 25,000 sperm around an egg. Both IUI and IVF potentially increase the risk of polyspermy and the likelihood of miscarriage.
Human fertilisation is a gigantic lottery with 250 million tickets: for healthy sperm, it is the luck of the draw
The possibility of polyspermy casts new light on the evolution of sperm counts. Discussions of sperm competition generally focus exclusively on maximising sperm counts, but – as is common in biology – some kind of trade-off is involved. Whereas natural selection can lead to increased sperm production if males are in direct competition, it will also favour mechanisms in the female tract that constrain numbers of sperm around the egg. In promiscuously mating primates, such as chimpanzees, increased oviduct length in females offsets increased sperm production by males. This presumably limits the numbers of sperm approaching the egg. It also shows that the female’s role in fertilisation is by no means as passive as is often assumed.
The entrenched idea that ‘the best sperm wins’ has elicited various suggestions that some kind of selection occurs, but it is difficult to imagine how this could possibly happen. The DNA in a sperm head is tightly bound and virtually crystalline, so how could its properties be detected from outside? Experiments on mice indicate, for instance, that there is no selection according to whether a sperm contains a male-determining Y-chromosome or a female-determining X-chromosome. It seems far more likely that human fertilisation is a gigantic lottery with 250 million tickets, in which – for healthy sperm – successful fertilisation is essentially the luck of the draw.
Other puzzling features of sperm also await explanation. It has long been known, for instance, that human semen contains a large proportion of structurally abnormal sperm with obvious defects such as double tails or tiny heads. The ‘kamikaze sperm’ hypothesis proposed that these dud sperm in fact serve different functions in competition, such as blocking or even killing sperm from other men. However, this has since been effectively discredited.
The entrenched notion that human sperm, once ejaculated, engage in a frantic race to reach the egg has completely overshadowed the real story of reproduction, including evidence that many sperm do not dash towards the egg but are instead stored for many days before proceeding. It was long accepted as established fact that human sperm survive for only two days in a woman’s genital tract. However, from the mid-1970s on, mounting evidence revealed that human sperm can survive intact for at least five days. An extended period of sperm survival is now widely accepted, and it could be as long as 10 days or more.
Other myths abound. Much has been written about mucus produced by the human cervix. In so-called ‘natural’ methods of birth control, the consistency of mucus exuding from the cervix has been used as a key indicator. Close to ovulation, cervical mucus is thin and has a watery, slippery texture. But precious little has been reported regarding the association between mucus and storage of sperm in the cervix. It has been clearly established that sperm are stored in the crypts from which the mucus flows. But our knowledge of the process involved is regrettably restricted to a single study reported in 1980 by the gynaecologist Vaclav Insler and colleagues of Tel Aviv University in Israel.
In this study, 25 women bravely volunteered to be artificially inseminated on the day before scheduled surgical removal of the womb (hysterectomy). Then, Insler and his team microscopically examined sperm stored in the crypts in serial sections of the cervix. Within two hours after insemination, sperm colonised the entire length of the cervix. Crypt size was very variable, and sperm were stored mainly in the larger ones. Insler and colleagues calculated the number of crypts containing sperm and sperm density per crypt. In some women, up to 200,000 sperm were stored in the cervical crypts.
Insler and colleagues also reported that live sperm had actually been found in cervical mucus up to the ninth day after insemination. Summarising available evidence, they suggested that after insemination the cervix serves as a sperm reservoir from which viable sperm are gradually released to make their way up the oviduct. This dramatic finding has been widely cited yet largely ignored, and there has never been a follow-up study.
Mutations accumulate four times faster in sperm than in eggs, so semen from old men is risk-laden
In his textbook Conception in the Human Female (1980) – more than 1,000 pages in length – Sir Robert Edwards, a recipient of the 2010 Nobel prize for the development of IVF, mentioned cervical crypts in a single sentence. Since then, many other authors have mentioned sperm storage in those cervical crypts equally briefly. Yet storage of sperm, with gradual release, has major implications for human reproduction. Crucially, the widespread notion of a restricted ‘fertile window’ in the menstrual cycle depends on the long-accepted wisdom that sperm survive only two days after insemination. Sperm survival perhaps for 10 days or more radically erodes the basis for so-called ‘natural’ methods of birth control through avoidance of conception. Sperm storage is also directly relevant to attempts to treat infertility.
Another dangerous misconception is the myth that men retain full fertility into old age, starkly contrasting with the abrupt cessation of fertility seen in women at menopause. Abundant evidence shows that, in men, sperm numbers and quality decline with increasing age. Moreover, it has recently emerged that mutations accumulate about four times faster in sperm than in eggs, so semen from old men is actually risk-laden.
Much has been written about the fact that in industrialised societies age at first birth is increasing in women, accompanied by slowly mounting reproductive problems. A proposed solution is the highly invasive and very expensive procedure of ‘fertility preservation’ in which eggs are harvested from young women for use later in life. However, increasing reproductive problems with ageing men, notably more rapid accumulation of sperm mutations, have passed largely unmentioned. One very effective and far less expensive and invasive way of reducing reproductive problems for ageing couples would surely be to store semen samples from young men to be used later in life. This is just one of the benefits to be gained from less sexism and more reliable knowledge in the realm of human reproduction.
Nowadays, the story of Hartsoeker’s homunculus might seem veiled in the mist of time, mentioned only as an entertaining illustration of blunders in the early exploration of human sex cells. But its influence, along with the macho-male bias that spawned it, has lived on in subtler form among the cultural stereotypes that influence the questions we ask about reproductive biology.